TLC may be either carried out by the adsorption principle (if the thin layer is prepared by an adsorbent such as “Keiselguhr” (or) “Alumina” (or) by the partition principle (if the layer is prepared by a substance such as “Silica gel” which hold water like the paper).
Preparation of the layer:
The glass plate should be washed thoroughly & dried before layer application. The material to be used for layer preparation is a follows:
The selected material is usually mixed with water, it form thick suspension, known as ”Slurry”. This slurry is applied to a plate surface uniformly with 0.25mm thickness. To this layer mix the binder “Calcium sulphate” for better adhesion of the stationary phase. The plates are dried after application of the slurry. If adsorption chromatography is to be performed, the thin layer is activated by heating at 1100 C for several hours.
|
Compounds |
Adsorbents |
Solvent systems |
| Mono and Disaccharides | Kieselguhr.G (Sod.acetate) Kieselguhr.G (Sod.Phosphate, pH-5.0) | Ethylacetate/Propanol (65/35) Butanol/Acetone/Phosphate buffer (pH-5.0) (40-50-10) |
| Amino acids | Silica Gel.G | 96% ethanol/water (70/30) |
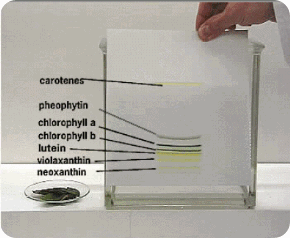
| Plant pigments | Kieselguhr.G | Petroleum ether/Propanol (99/1) |
Chromatographic plates (20X20cm) of 200m thicknesses are prepared by using a suspension of 30 grams of silica gel G in 63ml of 0.1M Na2CO3 solutions by shaking vigorously for 90 seconds. The silica gel slurry is applied on to the glass plate in the form of a uniform layer. These plated are activated at 1100C for 30 minutes immediately prior to use.
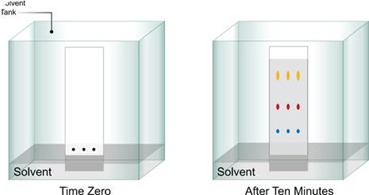
Then the samples (5 to 100mL) are applied on the silica plate in the form of small drops at regular intervals. In this plate these samples are applied as a spot of less than 5 minutes diameter on the lower right corner of the plates under a stream of warm air.
This plate is introduced into the saturated standard Brinkman developing chamber with the vapor of the solvent mixture with Chloroform: Methanol: Acetic acid: Water (250:74:19:3 v/v) to dip 4.5 cm of its bottom.
When solvent migrates about 15cm, plates are dried in air for 15 minutes and develop in the second dimension (900 rotation clockwise) with CHCl3: CH3OH: 7M NH4OH (230: 90: 50 v/v). The solvent front is again allowed to stand (or) more about 15cm. Then the plate is dried in air for 5 minutes and exposed to iodine vapor (or) UV light the sample molecules can be visualized. When a permanent record of developed plates is desired, plates are sprayed lightly with 10N H2SO4 and then heated at 1100 for 15 minutes.
By calculating the Rf values are can easily identified the molecules present in the mixture.
Detection: Several detection methods are available. They are,
1) Spraying the plate with 25 to 50% H2SO4 in ethanol and heating. This results in charring of most of the compounds, which show up as Brown spots.
2) Iodine vapours are used extensively as a universal reagent for organic compounds. This iodine spot disappears rapidly but can be made more permanent by spraying with 0.5% benzidine solution in absolute ethanol.
Applications:
1) The constituents of the mixture of amino acids, and the constituents of natural lipids and phospholipids are separated and estimated in a short time.
2) Enzymes, nucleic acids, pigments, sugars can also be separated by using this technique.
3) TLC has often been used to identify drugs, contaminants & Adulterants.
Advanced TLC:
TLC can be automated using forced solvent flow, running the plate in an vacuum-capable chamber to dry the plate, and recording the finished chromatogram by absorption or fluorescence spectroscopy with a light source. The ability to program the solvent delivery makes it convenient to do multiple developments in which the solvent flows for a short period of time, the TLC plate is dried, and the process is repeated. This method refocuses the spots to acheive higher resolution than in a single run. See for example: Poole, C. F.; Poole, S. K. "Instrumental Thin-Layer Chromatography," Anal. Chem. 1994, 66, 27A.
Two-dimensional TLC uses the TLC method twice to separate spots that are unresolved by only one solvent. After running a sample in one solvent, the TLC plate is removed, dried, rotated 90o, and run in another solvent. Any of the spots from the first run that contain mixtures can now be separated. The finished chromatogram is a two-dimensional array of spots.



I have been sourcing minerals for my production unit for over five years, but the consistency provided by Seema Minerals is truly unmatched. We recently switched to their premium grade kieselguhr for our filtration processes, and the results have been phenomenal. The purity levels are exactly as promised, which has significantly improved our end-product clarity. It is rare to find a supplier that understands technical specifications so well while maintaining competitive pricing. Their team is professional, and the delivery logistics are always on point. If you are looking for reliability and high-grade industrial minerals, I highly recommend giving them a try for your next project.
ReplyDeleteFinding a dependable supplier in the minerals industry can be a challenge, but Seema Minerals has exceeded all our expectations. Their high-porosity kieselguhr has become an essential component in our manufacturing workflow, offering excellent absorption and structural integrity. Since we started using their products, we have noticed a decrease in waste and an increase in overall efficiency. The customer support team is knowledgeable and helped us choose the right grade for our specific needs. For anyone in the market for top-tier minerals, this company is a standout choice. Their commitment to quality and transparency makes them a partner you can actually trust.
ReplyDelete