Paper Electrophoresis is one of the zone electrophoresis. This is very important method in all laboratories. In this article let us learn the details of the paper chromatography with suitable notes. I have given the info about this in Notes.
Principle:
“The charge carried by a molecule depends on the pH of the medium. Electrophoresis at low voltage is not usually to separate low molecular weight compounds because of diffusion, but it is easier to illustrate the relationship between charge and pH with amino acids than with proteins (or) other macromolecules”.
Filter paper:
Paper of good quality should contain at least 95% α-cellulose and should have only a very slight adsorption
capacity.
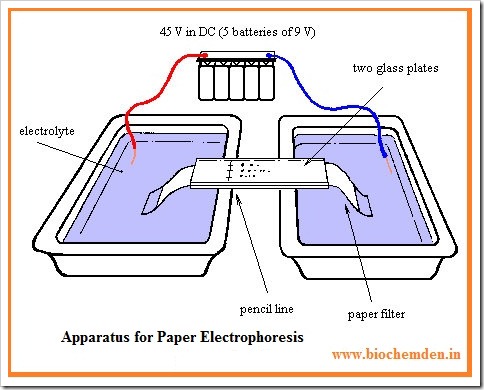
Apparatus:
- The equipment required for electrophoresis consists basically of two items, a POWER PACK and an ELECTROPHORETIC CELL.
- Power pack provides a stabilized direct current & has controls for both voltage & current out put, which have an out put of 0 to 500V and 0 to 150mA are available.
- The Electrophoretic cell contains the electrodes, buffer reservoirs, a support for paper and a supporting transparent insulating cover. The electrodes are usually made of platinum.
- The two arrangements of the filter strips are commonly used. The horizontal & vertical arrangements. Both the arrangements are equally viable & the choice usually depends upon personal preferences.
Sample application:
The sample may be applied as a spot (about 0.5cm in diameter) or as a narrow uniform streak.
Special devices are available commercially for this purpose. The sample can be applied before the paper has been equilibrated with buffer (or) after it.
Procedure:
After the sample has been applied to the paper and the paper has been equilibrated with the buffer.
The current is switched on. Commonly used buffers are,
The device providing stable voltage (or) current is available. Frequent observation is necessary to run electrophoretic apparatus. Overheating can be avoided by placing the entire equipment in the cold
room. The process does not take longer than two hours. After 2 hours switched off the power and paper is
removed. Once removed, the paper is dried in hot oven at 1100C.
Detection & Quantitative assay:
To identify the unknown electrophorogram, compare the Unknown electogram with standard electrogram under standard conditions.Individual compounds are usually identified by physical properties by the following methods.
i) Fluorescence:
a) Staining with “Ethidium bromide” and subsequent visualization of the electrophoreticgram under UV light makes DNA & RNA fluoresce and thus facilitates their detection.
b) Flourescamine staining is utilized for detecting amino acids, amino acid derivatives, peptides & proteins.
c) DANSYl chloride may be used in place of fluorescamine.
ii) UV absorption:
Proteins, Peptides & nucleic acids absorb in the range of 260 to 280nm, this property these can be detect.iii)) Staining:
iv) Detection of Enzymes in situ:
- If the component to be separated is an enzyme. Special techniques may be used to detect it.
- The paper strip, which have separated enzyme, is impregnated with the substrate for the enzyme desired to be separated.
- The paper strip is now placed in a suitable buffer along with electrophoretogram. The color bands will appear which indicates the position of enzyme.
v) Quantitative estimation:
The color density of the zone may be multiplied with the area of the zone and the resulting value would be a rough estimate of the concentration of the component.
Applications:
- Serum analysis for diagnostic purpose is routinely carried about by paper electrophoresis.
- Muscle proteins, egg white proteins, milk proteins & snake, insect venom analysis done by this technique.