Paper chromatography has proved to be very successful in the analysis of chemical compound and lipid sample in particular.
Nature of the paper:
The paper commonly used consists of highly purified cellulose. Cellulose, a homopolysaccharide of glucose. Contains several thousand anhydro-glucose units-linked through oxygen atoms. The paper exhibits weak ion exchange and adsorptive properties. Modified forms of paper have been produced in which the paper has been impregnated with alumina, silica gel, and ion-exchange resin etc.
The chemical composition of whatmann filter paper no: 1 is: a-cellulose (98 to 99%), b-cellulose (0.3 to 1%), Pentosans (0.4 to 0.8%), Ash (0.07 to 0.1%) & ether soluble matter (0.015 to 0.1%).
Apparatus:
1) Support for paper
2) Solvent trough
3) Airtight chamber
4) Whattmann filter paper number 1
5) Capillary tubes
6) Samples – Amino acids (or) Pigments
7) Solvents
8) Platinum loop
Paper development
There are two main techniques, which may be employed for the development of paper Chromatograms.
1) Ascending techniques
2) Descending techniques
3) Radial development
4) Two-dimensional chromatography
1) Ascending techniques:
The filter paper is then dried and equilibrated by putting it into on airtight cylindrical jar, which contains an aqueous solution of a solvent. The most widely applicable solvent mixture is n-butanol: acetic acid: Water (4:1:5), which is abbreviated as BAW.
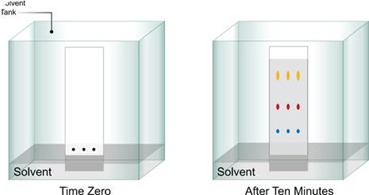
The sheet of paper is supported on a frame with the button edge in contact with a trough with solvent. The arrangement is contained in an airtight tank lined with paper saturated with the solvent to prove a constant atmosphere and separations are carried out in a constant temperature room. Thus, the solvent will ascend into the paper this process is, therefore, termed “Ascending Chromatography”
2) Descending techniques:
The end of the filter paper may be put into the solvent mixture contained in a narrow trough mounted near the top of the container. In this chromatography, the solvent will descend into the paper and this process is then termed “Descending Chromatography”.
This method is convenient for compounds, which have similar Rf values since the solvent drips off the bottom of the paper, thus giving a wider separation.
3) Two dimensional chromatography (3D):
The mixture is separated then the first solvent, which should be volatile: then after drying, the paper is turned through 900 and separation is carried out in the second solvent.
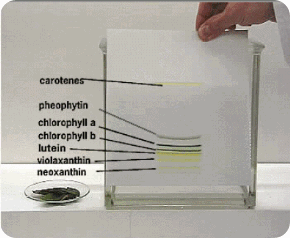
After locating the migrated unknown sample along with standard known sample, a map is obtained and comparing their position with a map of known compounds can identify compounds.
Locating the compounds:
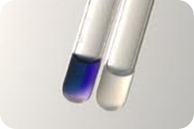
Strip is removed when the solvent has migrated over most of the available space. The distance to which the solvent has run is marked. In most cases, the completed Chromatogram is colorless with no indication of the presence of any compounds. Such a chromatogram is said as “Undeveloped” for locating the various compounds. The filter paper strip is first dried, then sprayed with 0.5% Ninhydrin in acetone and at least heated for a few minutes at 80 to 1000 C. the reaction occurs and the colored spots appear at the sites of the amino acids, such as Chromatogram is now called “Developed”.
In paper chromatography, the stationary cellulose phase is more polar than the mobile organic phase.
Identifying the compounds:
The ratio of the distance travelled by a component (i.e. amino acid) to that travelled by the solvent front, both measured from the marked point of the application of the mixture, is called the “Resolution front (Rf)” value for that component.
Distance from origin run by the compound
Rf = -----------------------------------------------------------------------------
Distance from origin run by the solvent
Detection:
The filter paper strip may be sprayed with ninhydrin and heated so that the colored spots indicating the location of amino acids may develop. The color densities of these spots may be measured with a recording transmittance (or) reflectance photometer device.
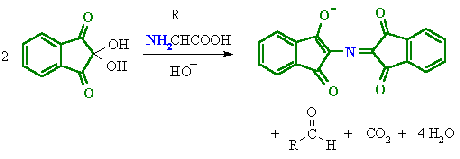
- Amines (including α-amino acids) react with ninhydrin to give a coloured product.
- It can be used qualitatively (e.g. for chromatographic visualisation) or quantitatively (e.g. for peptide sequencing).
- The α-amino acids typically give a blue-purple product.
- Proline, a secondary amine, gives a yellow-orange product.
- The test is sensitive enough that ninhydrin can be used for the visualisation of fingerprints.
Applications:
By using this technique
1) To check the control of purity of pharmaceuticals,
2) To the detection of adulterants,
3) To detect the contaminants in foods and drinks,
4) To the study of ripening and fermentation,
5) To the detection of drugs and dopes in animals & humans
6) To the analysis of cosmetics
7) To the analysis of the reaction mixtures in biochemical labs.